
Many proteins that are secreted from cells, or find themselves on the surface of cells, are "spot welded" in places along their length by crosslinks formed between two sulfur containing amino acid R-groups. The strongest of these intra-molecular forces is a covalent bond that forms, under the right circumstances, between the two R-groups of two cysteine amino acids. Intra-molecular forces attract or repel parts of segments of the amino acid chain as the various R-groups are twisted into proximity with one another. Many globular proteins, therefore, have their hydrophobic R-groups buried deep within their core, creating a water excluding region that plays a significant role in maintaining the overall three-dimensional structure of the final protein molecule.Ī different set of forces are at work within the polypeptide molecule itself. Just as molecules of lipid or hydrocarbon come together to form a droplet of oil or grease when placed in water, so do the hydrophobic R-groups, with much the same effect water is excluded and the whole structure stabilized. In doing so they create a zone or environment which is strongly water repelling. Many or most of the hydrophobic R-groups eventualy end up facing into the middle of the molecule which is the point furthest away from the surrounding water. Having these side chains surrounded by water would destabilize the protein molecule and make it very insoluble in water.įortunately the polypeptide molecules can reshape themselves in ways that prevent this from happening. Many of the R-groups sticking off a polypeptide chain are either hydrophobic or at least non-hydrophilic. Thus the protein does not "sink" to the bottom of the cell. The hydrophilic R-groups sticking out from the surface of the polypeptide/protein interact with the water molecules and hold the huge macromolecule in suspension.
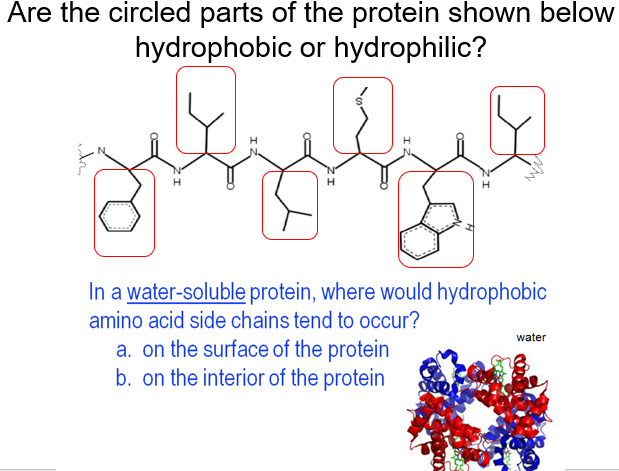
This has two effects on the polypeptide/protein macromolecule it starts to give the whole molecule a characteristic shape, and also provides a means of support. When in an aqueous environment, the polypeptide bends and twists until the maximum number of hydrophilic R-groups are extended out into the water where they are stable. One of the most important of these forces is the action and interaction of the hydrophilic acidic and basic R-groups and the surrounding water.
#Hydrophobic amino acids in a protein series
This shape is not random, but the result of a series of different inter- and intra-molecular forces between the inner R-groups, the peptide bonds, and the outer watery environment. However, because the polypeptide is a flexible chain, it can bend and twist itself into almost any shape. When a polypeptide is formed in water, therefore, it would not be able to play any kind of biological role at all if it was pulled at once, by gravity, to the bottom of the cell and left there! Proteins are very large molecules which are certainly heavy enough to sink to the bottom of a cell if not supported. When in water, most polypeptides spontaneously fold themselves into a shape that is both stable and critical for the biological role it is about to play. Since the backbone of the polypeptide, held together by peptide bonds, is flexible (because of the "rotation about all those bonds"), the chain can bend, twist, and flex into a very large variety of three dimensional shapes. This property of "rotation about a bond" has important consequences for all the other properties of polypeptides and the proteins. The covalent bonds that hold them together allow the atoms to rotate and take up a three-dimensional position in the molecule where they are the most stable. These R-groups are neither strongly hydrophilic nor hydrophobic.Ītoms in long molecules, such as polypeptides, are not rigidly fixed in space or position. They are hydrophobic.Ībout 5 amino acids have polar side chains, R-groups which do not ionize or become positively or negatively charged. There are about 10 nonpolar amino acids with R-groups that are not stable when in contact with water.
These types of R-groups are strongly hydrophilic and are stabilized when surrounded by water. There are about 5 amino acids that are either acidic or basic, and their R-groups ionize at various pHs to produce a chemical structure that has either a positive or negative charge. Amino acids, polypeptides and flexible chainsĪll proteins consist of long chains of amino acids joined in a sequence that has been determined by the information stored in DNA genes and interpreted by the transcription and translation machinery of the cell.Įach amino acid carries a side chain (or R-group) that can, in theory, take a lot of different chemical forms, but only 20-22 of these forms are found in common proteins.ĭespite their individual chemical differences, amino acids (and their R-groups) can all be put into four different "families" depending on whether their R-groups are:


 0 kommentar(er)
0 kommentar(er)
